Corpus Luteum
Authors
INTRODUCTION
The corpus luteum is a remarkable, transiently functioning organ that provides the endocrine conditions that are necessary and sufficient for the establishment and maintenance of early pregnancy. On a weight basis, it is the most productive steroid-secreting tissue in the body. It is abruptly formed from the remnants of the preovulatory follicle, and it undergoes continuous change thereafter. This chapter presents the corpus luteum in the context of its changing properties over time in the early, middle, and late luteal phase in cycles without conception and during early and late pregnancy. Themes for understanding the corpus luteum center on the sudden induction of an immense steroidogenic capacity, the importance of new vessel formation to this process, and the supervening effects of apoptosis in the event of conception failure.
PERIOVULATORY EVENTS: TRANSFORMATION OF THE FOLLICLE TO THE CORPUS LUTEUM
The corpus luteum is formed by the action of luteinizing hormone (LH) on the mature preovulatory follicle. Ensuing events, including steroidogenic outpouring, programmed senescence, and capacity for extension by gestation, depend on features characteristic of an adequately developed preovulatory follicle, including, most importantly, the number and LH receptivity of the granulosa cell population, both of which are follicle-stimulating hormone (FSH)–dependent properties. Acquisition of LH receptor (LHR) by preovulatory granulosa cells results from estrogen-stimulated and FSH-stimulated transcription of the LHR gene, the actions of which are mediated largely by intracellullar cyclic adenosine monophosphate (cAMP).1,2 Actions of LH and human chorionic gonadotropin (hCG) define the functional unfolding of luteal events and are thought to be exclusively dependent on activation of this single, G-protein–coupled receptor. LHR-mediated effects occur primarily via the Gs/adenylyl cyclase/cAMP/PKA signaling pathway, although evidence for activation of other signaling pathways (e.g., inositol phosphate pathway) and possible roles for these is accumulating.3 The luteotropic action of LH or hCG on follicles that are not mature can produce luteinization of the theca, where LHRs are present, but does not set in motion the distinctive sequence of changes in morphology and function of the granulosa and theca that characterize the corpus luteum.
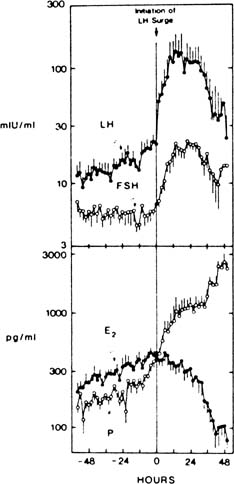
In ovulatory cycles, there is an increase in progesterone levels that begins before the LH surge, thought possibly to contribute to the positive feedback signal for pituitary release of LH (Fig. 1).4,5,6,7 This harbinger of luteal function is accompanied by a preovulatory increase in circulating 17-hydroxyprogesterone, the levels over time of which differ in pattern from those of progesterone by decreasing after the LH peak, in contrast to the rapid postovulatory increase in progesterone levels.5,8 These events may reveal an innate tendency for the granulosa cells of the mature follicle to secrete progestins, as occurs spontaneously when such cells are studied in vitro without an LH signal.9 They foretell the remarkable increase in the overall rate of steroidogenesis that soon will be under way: In a few days, the steroidogenic output of the ovary increases from a few hundred micrograms of estrogen to 20 mg or more of progesterone daily—a 100-fold increase. Ovulation marks a shift in inhibin production from the inhibin B dominance of the follicular phase to predominant production of inhibin A, the levels of which roughly parallel steroid secretion by the corpus luteum. Late follicular phase granulosa cells elaborate alpha subunit and beta (B) subunit mRNA in response to FSH and LH.10
The increase in steroidogenesis in the periovulatory period is accompanied by granulosa cell proliferation and considerable functional and structural reorganization that eventuate in the distinct morphology of the corpus luteum. Expression of the transcription factor early growth response factor-1, known as a coordinator for multiple transcriptional alterations in circumstances of tissue change, is induced in human granulosa cells by hCG. Expression of this growth factor also is stimulated by cholinergic activation of muscarinic receptors on granulosa cells.11 Activation of muscarinic receptors also blocks gap junctions via phosphorylation of connexins therein and stimulates cell proliferation via increases in intracellular calcium, together suggesting that cholinergic mechanisms may participate in the rapid reprogramming of granulosa cells in the periovulatory period.12
CORPUS LUTEUM OF THE EARLY LUTEAL PHASE
Histology and Ultrastructure of the Early Corpus Luteum
During the first several days after ovulation, there are important changes in histoanatomy, histology, and ultrastructure that describe the transformation of the mature follicle into the corpus luteum.4,13,14,15 At first, there is little to distinguish the histology of the corpus luteum from that of the late preovulatory follicle.4,14 The granulosa cells are not yet luteinized, or lipid-laden, and this layer remains avascular. Theca cells remain large and lipid-laden, and vessels of the theca are engorged. There may be a small amount of bleeding into the central cavity and, in early specimens, this feature may be the chief clue that ovulation has occurred.
Mitoses in the granulosa layer are seen in this early phase, and luteinization of granulosa cells (enlargement and accumulation of intracellular lipid) begins in earnest. Ultrastructurally, this change in granulosa lutein cells is accompanied by an accumulation of cytoplasmic lipid droplets, the appearance of a well-developed granular endoplasmic reticulum, and tubular cristae in the mitochondria—all changes typical of actively steroidogenic cells. There is organization of the cell into a peripheral zone of tubular endoplasmic reticulum, with few lipid droplets and mitochondria, and a central zone of mitochondria and well-dispersed Golgi, often with intervening parallel arrays of rough endoplasmic reticulum.13,15,16,17 This geographic arrangement may allow for accession and presentation of cholesterol from the lateral cell border to the mitochondria and for steroid to be passed to the Golgi.16,17
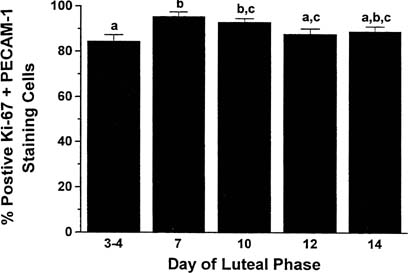
The transformation of the follicular granulosa cells to the granulosa-lutein cell mass of the corpus luteum is accompanied by the formation of new vessels that develop rapidly over the next few days. The formation of new vessels supports luteal function and export of luteal products and is mediated by vascular endothelial growth factor (VEGF), acting in a paracrine fashion.18,19,20 Locally elaborated VEGF drives endothelial cell proliferation, and endothelial cells comprise more than 50% of the cell population of the corpus luteum.21,22 Cell proliferation in the corpus luteum is limited almost exclusively to vascular endothelial cells (85% of mitotically active cells are endothelial cells) and is greatest in the young corpus luteum (Fig. 2).21,23,24
Steroid Secretion by the Early Corpus Luteum
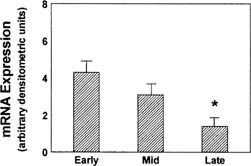
Development of the extraordinary steroidogenic productivity of the corpus luteum is realized within a few days of ovulation. Acquisition of substrate is accomplished by low-density lipoprotein (LDL) receptor–mediated binding and internalization of very-low-density lipoprotein–associated cholesterol.25,26,27,28 Cholesterol molecules thus acquired next must reach the inner mitochondrial membrane, a step mediated by the sterol transfer protein StAR (steroidogenic acute regulatory protein).29,30,31 Finally, at the inner mitochondrial membrane, cytochrome P-450 side-chain cleavage (P-450scc) enzyme effects the synthesis of pregnenolone from cholesterol. LH acts via the cAMP second messenger system, in synergy with insulin, to enable each of these three key steps: increased expression of LDL receptors, expression of StAR protein, and of P-450scc enzyme.32,33 Expression of StAR is mediated by the transcription factors steroidogenic factor 1, CCAAT/enhancer binding protein beta, and other as yet uncharacterized transcription factors and coregulators.31 StAR mRNA expression is greatest in the newly formed corpus luteum (Fig. 3).34 Pregnenolone, the immediate product of side-chain cleavage of cholesterol, is transformed into progesterone by the enzyme 3β-hydroxysteroid dehydrogenase, Δ 4-5-isomerase located in smooth endoplasmic reticulum. The transport of newly formed progesterone from the cell is thought to be a process of passive diffusion35; however, an active secretory mechanism for steroidogenic cells has been proposed.36,37
Progesterone concentrations are 10-fold higher in the peritoneal fluid than in peripheral blood in the days immediately after ovulation, probably as a result of impeded vascular export from the corpus luteum.38 The rate of secretion through the peritoneal compartment may be substantial because there is ample vascularized surface area for absorption, but progesterone entering this compartment is largely metabolized before reaching the peripheral circulation because of the hepatic first-pass effect of the splanchnic circulation.39 Peritoneal fluid levels of progesterone decrease as the luteal phase advances, presumably as more steroid finds its way into the circulation directly through newly forming vascular channels in the corpus luteum. This interpretation is supported by the finding that the maximum tissue levels of progesterone are found in the early corpus luteum and decline thereafter.40
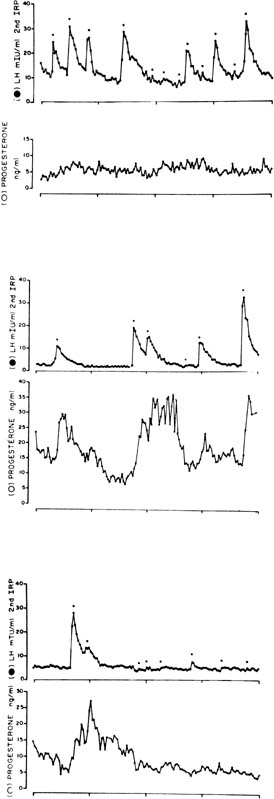
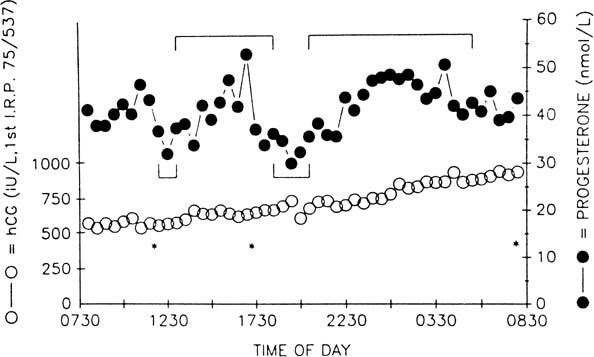
The pattern of progesterone secretion into the bloodstream by the early corpus luteum is dominated by a basal or tonic secretion, with little in the way of a pulsatile response to the episodic secretion of LH by the pituitary (Fig. 4).41 Progesterone secretion of the early corpus luteum is augmented little by hCG administration.42,43,44 This picture correlates with the functional predominance of the larger of the two functional and morphologic cell types identified in vitro in preparations of dispersed luteal cells.
Isolated luteal cells studied in vitro can be grouped into two populations, determined by size, that exhibit different secretory physiology.44,45,46,47 The large cells possess a higher basal rate of progesterone secretion than the small cells, but they are indifferent to hCG stimulation, are devoid of LHRs, and show enhanced estrogen biosynthesis when stimulated by FSH. By contrast, hCG markedly increases progesterone synthesis by small luteal cells, which do have LHRs, but in which estrogen secretion is not enhanced by FSH. These two cell types exhibit functional parallels with the theca and granulosa cells of the preovulatory follicle and may correspond to the granulosa-derived and theca-derived components of the corpus luteum.16,44,45
In vivo, the overall function of the corpus luteum is LH dependent, as shown by prompt luteolysis after administration of antisera to LH47 or gonadotropin-releasing hormone (GnRH) antagonists or agonists48,49,50,51 or after withdrawal of pulsatile GnRH in GnRH-dependent individuals.52,53 Clinically, when ovulation is induced with pulsatile GnRH for hypothalamic amenorrhea, maintenance of luteal function requires continued, pulsed GnRH administration or administration of hCG.54,55
Peptide Secretion by the Early Corpus Luteum
Inhibin has been identified as a secretory product of luteal cells in vitro.56,57 Levels of inhibin A begin to rise in the late follicular phase, peak in the mid luteal phase, and decline with luteal demise. Inhibin B exhibits high levels in the circulation in the late follicular phase and early luteal phase, and these decrease thereafter. Circulating levels of inhibin during the luteal phase are accounted for by the corpus luteum58 and roughly parallel progesterone levels.59 Granulosa luteal cells express mRNA for the inhibin alpha subunit to a greater extent than the mRNAs for beta A and beta B subunits, which is consistent with luteal production of inhibin A, inhibin B, and free inhibin alpha subunit.60,61 The LH-hCG dependence of luteal inhibin secretion has been confirmed in vitro56 and in vivo.49 The luteal phase depression of FSH levels may reflect in part the FSH-lowering effect of inhibin.62 This function would serve to avert the chaotic consequences of the superimposition of active folliculogenesis on luteal function.63 In addition to modulation of gonadotropin secretion, inhibin may exert paracrine influences on luteal function and development.
Abnormalities of the Early Corpus Luteum
The postovulatory rise in progesterone levels is delayed in some women, constituting a specific defect in the onset of luteinization. This special form of luteal dysfunction has been associated with a lag in the progression of secretory change in the endometrium and infertility.64,65,66
Hemorrhage into the central cavity of the corpus luteum is seen normally, but occasionally it can be excessive and warrant surgical attention because of pain or hemoperitoneum. Such clinical events can occur at any time during the life span of the corpus luteum of the cycle and during early pregnancy. Anticoagulant therapy is the sole known antecedent for these rare, otherwise sporadic events.67
CORPUS LUTEUM OF THE MID LUTEAL PHASE
By the mid luteal phase, the blood levels of progesterone, estradiol, and inhibin have reached their maxima, and 17-hydroxyprogesterone levels achieve a second peak.8 Now, at the full expression of its secretory capacity, the corpus luteum is poised at the junction of a course leading to regression and demise, in the nonpregnant cycle, or rescue and extended function, should pregnancy occur.
Histology and Ultrastructure of the Corpus Luteum of the Mid Luteal Phase
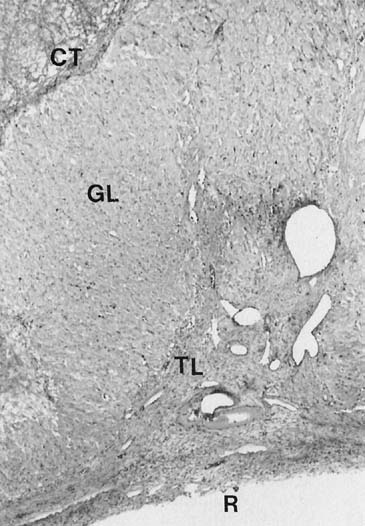
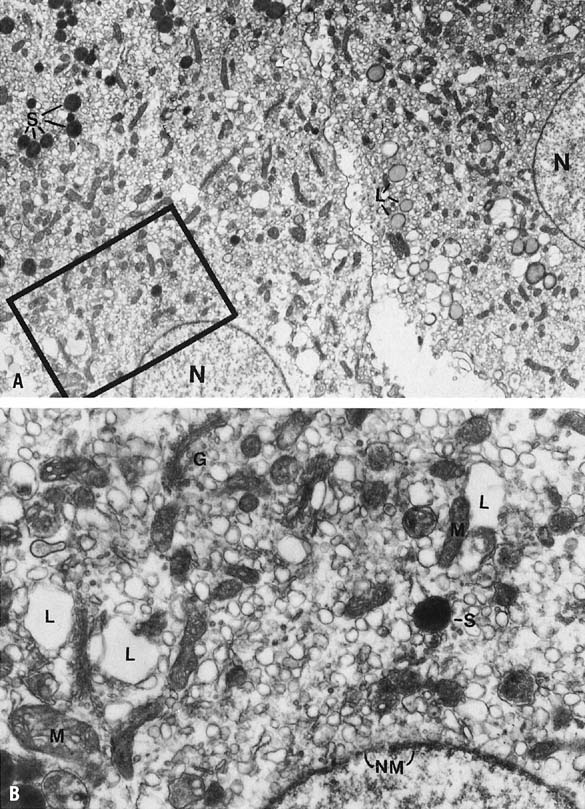
Histologically the granulosa layer is fully luteinized, festooning the margin of organelle (Fig. 5). Central hemorrhage becomes isolated from the underlying granulosa by an organizing layer of fibroblasts. Fresh bleeding into the central cavity still is seen commonly in surgical specimens. Theca cells have regressed except in regions where they have invested infolding septae, the theca lutein cells.13,14,68 Small darker cells, the so-called K cells, correlate ultrastructurally with perivascular macrophages.16 Distinctive ultrastructural features of active granulosa lutein cells now include extensive development of granular endoplasmic reticulum,69 microfilaments investing the cytoplasmic periphery bordering intercellular and intracellular canaliculi,17 and patches of microvilli that protrude into the perivascular space.16 These modifications suggest enhanced absorptive capacity, probably for cholesterol. Areas of granular endoplasmic reticulum also are seen and may reflect synthesis of peptides such as relaxin and inhibin (Fig. 6).16
Steroid Secretion by the Corpus Luteum of the Mid Luteal Phase
The progesterone secretory response of the corpus luteum to exogenous hCG is maximal at this luteal stage.42,43,44 Correspondingly the LH pulse–dependent transient increases in progesterone levels become more pronounced, and basal secretion is proportionately less important (see Fig. 4).41 This evolution in secretory response to LH and hCG may reflect an increasing functional predominance of the smaller luteal cell type seen in vitro70 and foretells the hCG responsiveness that will be required of the corpus luteum in the event of conception and implantation. StAR mRNA transcripts are diminished only slightly relative to the high levels of the early corpus luteum, whereas StAR protein abundance remains at least as great (see Fig. 3).34 Secretory bursts of progesterone occur with decreasing frequency, reflecting the decreased LH pulse frequency resulting from the effect of progesterone on the hypothalamic GnRH pulse generator (see Fig. 4).71 Expression of the function of the mature corpus luteum depends on the vascular investment of the organelle. Luteal function at this stage is compromised if VEGF-mediated angiogenesis is impaired.72 Expression of VEGF and its receptor, flt-1, remain as high as in the early luteal phase.20
Peptide Secretion by the Corpus Luteum of the Mid Luteal Phase
Inhibin B levels have started a decline by the midluteal phase, whereas inhibin A levels reach their maximum. In vitro inhibin production by dispersed luteal cells is stimulated by hCG, but not FSH, and more so in the early and mid luteal phase than in late luteal phase tissue.73 Inhibin A infused during the mid luteal phase reduces levels of FSH, without altering LH or progesterone levels or altering luteal phase length.62
Hypothalamic-Pituitary-Luteal Regulation
The regulatory relationship of corpus luteum function to the trophic influence of LH is poorly understood. The corpus luteum remains dependent on LH (or hCG) throughout its life span, and progesterone in turn modulates gonadotropin secretion, most notably by effecting a decrease in the frequency of gonadotropin pulses as the luteal phase evolves.71 This effect can be attributed to progesterone-mediated increase in opioid levels affecting the medial basal hypothalamus and the GnRH pulse generator.74,75 Progesterone and inhibin also may affect the secretion of gonadotropins directly at the level of the pituitary. In contrast to the relation of most endocrine glands to their trophic support, however, there is little evidence of an active feedback loop for luteal secretion and secretion of gonadotropins. In short-term experiments, exogenous augmentation of progesterone levels does attenuate LH secretion.75 Luteal phase progesterone levels in spontaneous cycles correlate only weakly, however, if at all, with mean LH levels.76,77 In particular, there is no evidence for a compensatory increase in LH secretion when progesterone levels are low in defective luteal phases77 or after luteectomy.78
Functional Abnormalities of the Corpus Luteum of the Mid Luteal Phase
The lower limits of normal for progesterone levels during the mid luteal phase are not well defined. Progesterone levels are frequently normal when measured during defective luteal phases, defined in terms of delayed secretory transformation of the endometrium. When carefully studied, integration of progesterone levels does correlate with clinically defined luteal adequacy, although a troubling overlap of normal and abnormals persists.77 Mid luteal inhibin A levels are decreased in women with luteal phase deficiency, defined by diminished mid luteal progesterone levels.79 Mid luteal inhibin A and immunoreactive inhibin alpha subunit are not diminished among women with luteal deficiency defined by endometrial maturation who have normal mid luteal progesterone levels.80,81 The poor correlation of peripheral progesterone levels with other determinants of luteal function may rest in the unreliability of single samples in representing the episodically variable levels of the hormone and the problem of accounting for its extensive, perhaps variable plasma binding to cortisol binding globulin and albumin.65
LATE LUTEAL PHASE: LUTEAL REGRESSION
By the 10th to 11th postovulatory day, progesterone secretion declines despite continued LH support. Now, LH pulses occur at lower frequencies than previously, and functional luteolysis has started. Functional luteolysis can be defined as the progressive decline in the capacity of the corpus luteum to respond to LH and hCG. Without rescue by hCG, progesterone secretion now becomes negligible over the next 2 to 3 days. This process can be overcome only by the timely and rapid increase in trophic support generated by an hCG from a successfully established gestation. Luteolysis is an essential conclusion to the nonconception cycle: It permits re-establishment of conditions supporting renewed folliculogenesis and endometrial regeneration.
Histology and Ultrastructure of the Regressing Corpus Luteum
Histologic evidence of regression includes shrinkage of cells in the granulosa layer. Theca and granulosa cells are progressively vacuolized, accounting in the former for the distinctive appearance of so-called mulberry cells.14 Histochemical studies show rapid loss of key steroidogenic enzymes.82 Ultrastructurally, there is breakdown of the organization of the endoplasmic reticulum, Golgi regression, and appearance of lipid droplet inclusions.13,69 The lutein zone exhibits progressive fibrosis, which presages ultimate hyalinization. Connective tissue organization of the central cavity continues, but even in the latest specimens fresh hemorrhage often is encountered.
Steroid Secretion by the Regressing Corpus Luteum
Progesterone secretion by the regressing corpus luteum increasingly is accounted for by episodic bursts in response to LH pulses.41 LH pulse frequency is at its lowest ebb; sometimes only a handful of pulses are seen in 24 hours. Progesterone peak amplitudes decline, and because basal secretion is now negligible, progesterone levels fall to preovulatory values in the long pulsatile intervals (see Fig. 4).41
Peptide Secretion by the Regressing Corpus Luteum
Inhibin secretion declines in a pattern parallel to that of progesterone.59,83,84 Declining steroid and peptide levels lead to increased GnRH pulse frequency and increased secretion of FSH, allowing initiation of the following cycle. Release from the inhibition of inhibin A does not seem to be as important as the effect of decreasing levels of estradiol in allowing re-emergence of FSH secretion during the transition to the follicular phase.85 Estradiol suppresses the increased inhibin B levels observed after menses, and the increase in levels of inhibin B and estradiol at the initiation of the next cycle depends on the increased gonadotropin pulse frequency that occurs after menses.86
Mechanism of Luteolysis and Apoptosis
Part of the decline in luteal function may be accounted for by the increasingly desultory secretion of LH in the late luteal phase,87 but experimentally induced decreases in LH pulse frequency88 or amplitude89 alone do not cause luteal failure. The progressive refractoriness of the corpus luteum to LH stimulation is the essential expression of luteolysis. When the cycle has ended, little if any progesterone secretion can be evoked from the regressed corpus luteum, even by large doses of hCG.42,70,90
Refractoriness to LH stimulation in vivo is reflected in in vitro studies. A decline in available LHR sites accompanies luteolysis,91,92,93 but it is not clear whether this is a causal or a secondary event.94 The declining secretory response to LH and hCG is accompanied by a decreasing secretory response to cAMP and its analogues, evidencing a role for postreceptor changes in functional luteolysis. Reduced transcription of StAR protein mRNA and dwindling expression of StAR protein occur and are likely an important component of the functional decline of the late corpus luteum (see Fig. 3).34,95
Prostaglandin F2α (PGF2α) is well established as the luteolysin in some species where it originates in the endometrium and traverses the utero-ovarian vasculature to reach the corpus luteum.46,96,97,98 PGF2α abrogates LH stimulation of cAMP production in vitro,99,100 possibly by decreasing LHR numbers101,102,103 or by actions downstream in the receptor/second messenger cascade.104 PGF2α suppresses basal and gonadotropin-stimulated StAR levels and stimulates expression of DAX-1, a negative transcription factor that reduces StAR promoter responsiveness to cAMP.105 A luteolytic effect of PGF2α acting to decrease perfusion of the corpus luteum also has been proposed, and vascular regression is observed in the aging corpus luteum.22,24,106 The stimuli for angiogenesis fade; mRNA and protein expression for VEGF and its receptor, flt-1, are diminished in the corpus luteum of the late luteal phase.20 PGF2α originating from the nonpregnant secretory endometrium is not the luteolysin in the primate, in which a normal luteal span is seen in hysterectomized primates.107,108,109 Data from humans and nonhuman primates support a luteolytic role for intraluteally generated PGF2α,40,99,110,114 and luteolysis can be evoked in nonhuman primates by intraluteal infusion of PGF2α115 Peripheral infusion of PGF2α does not cause luteolysis,116,117 however, perhaps because these compounds are metabolized rapidly in the pulmonary circulation. Prostaglandin synthesis inhibitors do not override luteolysis118,119 or extend the luteal phase, an observation explained by the known luteotropic influence of prostaglandins (e.g., prostaglandin E) other than PGF2α.120,121
Estrogen has a luteolytic effect, in vivo and in vitro,103,122,123,124,125 and the estrogen content of regressing corpora lutea is higher than at earlier stages.126 The direct luteolytic action of estrogen seen in in vitro observations may result from estrogen-induced increases of PGF2a production by luteal cells.115,127In vivo effects of estrogen on luteal function also may include estrogen inhibition of LH secretion. High luteal-phase estrogen levels may account for luteal defects in some instances of induced ovulation54,128 and ovarian hyperstimulation.129
Regression of the corpus luteum is accompanied by programmed cell death, or apoptosis.130 This universal metazoan mechanism for eliminating unneeded cells depends on the release and activation of specific, mitochondrially sequestered enzymes that include apoptosis-inducing factor and serine proteases known as caspases.131 Caspase activation by proapoptotic proteins such as Bax and inhibition by antiapoptotic signals such as Bcl-2 parallel observed apoptosis. Bcl-2 expression and mRNA are high in the mid luteal phase and diminished in the regressing corpus luteum, whereas Bax is most prevalent in the regressing corpus luteum.132
Functional Abnormalities During Luteal Regression
Luteal function can be abnormally short in duration, reflecting premature or accelerated luteolysis, and this can lead to infertility and perhaps recurrent pregnancy loss.133 Lower progesterone levels described throughout the luteal phase in short luteal-phase cycles suggest a global deficiency of luteal physiology, rather than a simple truncation of normal events.134,135 Several studies implicated abnormalities of gonadotropin secretion during preceding follicular phase in the genesis of the shortened luteal phase.77,134,135,136,137,138,139
Abnormal extension of luteal function also has been described. Termed Halban’s syndrome, the clinical picture is one of recurrent, functioning cystic corpora lutea, with abnormal vaginal bleeding. It is possible that clinical events attributed to this diagnosis occurred because of early, failing intrauterine or clinically occult tubal pregnancies. Recurrent, nongestational extension of luteal function has been documented rarely since the advent of sensitive blood and urinary hCG assays.140
CORPUS LUTEUM OF EARLY PREGNANCY: LUTEAL RESCUE
In the event of conception and implantation, rising levels of hCG originating from the trophoblast transform the corpus luteum histologically and morphologically. There are quantitative and qualitative changes in secretory activity, which include increased and smoothed secretion of progesterone (owing to the nonsaltatory nature of hCG levels), increasing secretion of inhibin, and the onset of secretion of relaxin. A new wave of vascular endothelial proliferation in the corpus luteum occurs in response to the rising hCG of early pregnancy.22,24
Histology and Ultrastructure During Early Pregnancy
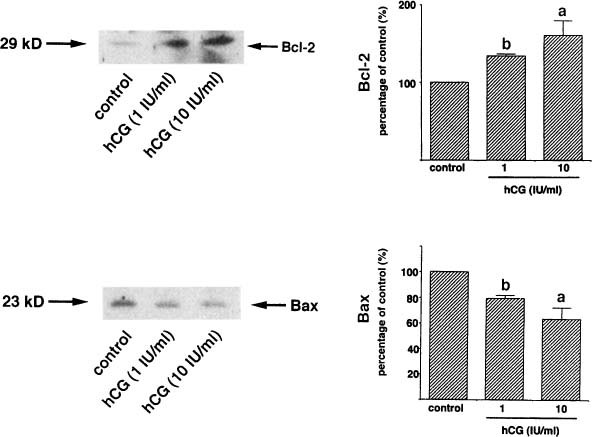
The corpus luteum generally is enlarged, principally by virtue of an increased volume of the central cavity.141 Connective tissue surrounding the corpus luteum becomes hyperplastic and extensively vascularized. The theca luteum layer becomes prominent, and this zone appears to be the site of early cellular response to hCG.142 Ultrastructurally, functional cells resemble those of the midluteal phase, but with an accentuation of the compartmentalization of organelles.13 Apoptosis, characteristic of the regressing corpus luteum of the nonpregnant cycle, is virtually inapparent in the corpus luteum of early pregnancy. The absence of apoptosis is paralleled by sustained levels of Bcl-2, characteristic of the midluteal corpus luteum, and absence of the expression of Bax, which is seen in the regressing corpus luteum.133 These alterations may reflect the influence of hCG, which increases the mRNA and protein levels of Bcl-2 and decreases those of Bax when midluteal cells are incubated in vitro (Fig. 7).133
Steroid Secretion by the Corpus Luteum of Early Pregnancy
In early pregnancy, progesterone secretion and that of estradiol and 17-hydroxyprogesterone slightly exceed that of the nonpregnant mid luteal phase.143,144,145,146 The mechanism of the hCG-induced abrogation of luteolysis is incompletely understood but may include modulation of intraluteal prostaglandin synthesis.147,148 The enhancement of progesterone secretion by rising hCG is seen from near the time of implantation onward,145 but progesterone secretion seems to increase slowly, if at all, thereafter. The gradual increase in levels of progesterone in early pregnancy is accounted for more readily by increasing progesterone secretion by the trophoblast.149 The corpus luteum can be estimated to account for approximately 76% of the circulating progesterone at 6 weeks, 50% by 10 to 11 weeks, and 33% by 14 to 15 weeks.150 The decreasing contribution of the corpus luteum to circulating progesterone also is reflected by the decline in blood levels of 17-hydroxyprogesterone, exclusively a luteal product, relative to the gradually rising progesterone levels of early pregnancy.144,151 The studies of Csapo and colleagues definitively delineated the indispensability of the corpus luteum to early pregnancy maintenance until about the seventh week152 and determined that this essential role could be accounted for entirely by progesterone.149,153,154 The evolution of gestational dependency on the corpus luteum to independence was called the luteoplacental shift and seems to correspond to the ability of the trophoblast to maintain progesterone levels at levels exceeding approximately 5 ng/mL.
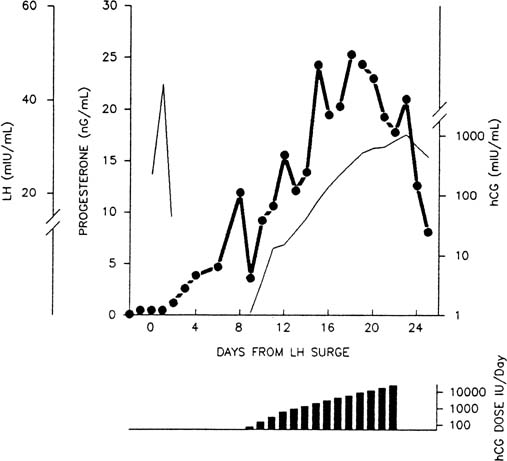
The modestly enhanced, but relatively constant, luteal output of progesterone in early pregnancy is accounted for by hCG. The dramatic rate of rise of hCG levels seen in early gestation seems essential to the maintenance of progesterone secretion.150,155 Progressive refractoriness to hCG at fixed doses is found in vivo156,157 and in vitro.70 By contrast, administration of hCG in increasing doses—in a manner mimicking early pregnancy—results in extension of luteal function (Fig. 8).42,158
Failure of decreasing or inadequately increasing hCG levels to sustain luteal progesterone production in early pregnancy can be attributed to desensitization of the granulosa cells. Desensitization of LHR function has been shown in vitro to include (1) internalization of receptor down-regulation and (2) uncoupling of the receptor from functional interaction with its associated G-protein. Both features of this post-transcriptional modulation of receptor have been shown to occur as a result of receptor phosphorylation (an accompaniment of G-protein activation) and subsequently are mediated by beta-arrestin proteins that bind the phosphorylated receptor.3,159 This process may explain how the smoothly increasing levels of hCG as opposed to episodic levels of LH may facilitate the striking and progressive refractoriness of the corpus luteum of early pregnancy to hCG support. Clinically, this phenomenon is reflected by the low progesterone levels seen when hCG levels fail to increase normally in early pregnancy. When pregnancies with abnormally rising hCG are intrauterine, resulting progesterone secretion is low, effecting inadequate decidual support and spontaneous abortion. Because low progesterone levels on a single determination are a marker for poor hCG rise, spot progesterone levels have utility as a screen for ectopic pregnancy.160
The progressive refractoriness of the corpus luteum to LH and hCG that constitutes functional luteolysis seems to extend into pregnancy and is overcome only by the rapid rate of rise of hCG. This refractoriness may be a result of a decrease in LH-hCG receptor number or affinity161; increased hCG receptor occupancy has been documented in early pregnancy.92 Levels of hCG do not vary, as do those of LH with its episodic secretion and shorter half-life; this is a likely explanation for the fact that the short-term variation in levels of progesterone characteristic of the late luteal phase is reduced in early pregnancy. Substantial short-term variability in progesterone levels remains, however, and may be accounted for by short-term variation in volume of distribution and metabolism of the hormone (Fig. 9).162
Peptide Secretion by the Corpus Luteum of Early Pregnancy
Relaxin, a polypeptide with homologies with insulin and nerve growth factor, is produced by the corpus luteum of early pregnancy. It circulates in small quantities in the final days of the nonpregnant cycle,163 but this increase is accelerated and continued in cycles of conception under the influence of hCG, independent of hCG stimulation of progesterone secretion.164,165,166 It can be evoked by the administration of hCG late in the luteal phase of a nonpregnant cycle,167 and levels in multiple pregnancy are related to the number of concepti.168 In early pregnancy, corpus luteum volume correlates more closely with hCG and relaxin levels than levels of progesterone, and relaxin levels correlate with levels of hCG.169 Relaxin increases expression of VEGF by endometrial cells in vitro and may play a role in endometrial neovascularization.170 In many species, relaxin induces remodeling of connective tissue of the birth canal and inhibits myometrial contractility.171 Known nonreproductive effects of relaxin, of unclear significance to the physiology of human pregnancy, include cardiovascular, breast, antiplatelet, and pituitrophic activities.172 Placenta and decidua are sites of synthesis of relaxin as well, which may explain higher levels of relaxin observed in pregnancies in diabetic women.173,174 Aluteal pregnancies achieved through assisted reproduction techniques are relaxin deficient, without apparent consequences to implantation, pregnancy maintenance, or mechanism of labor and delivery.166,175,176,177 Without an essential function in human pregnancy, the time-dependent and signal-dependent luteal secretion of relaxin points to ongoing differentiation of function within components of the corpus luteum long after it is formed.
Expression of inhibin alpha subunit and beta A subunit continues in the corpus luteum of early pregnancy.61 Also there is evidence that under the influence of hCG, the corpus luteum is the source of the increase in circulating prorennin observed early in cycles of conception.178
Functional Abnormalities of the Corpus Luteum of Early Pregnancy
Sporadic and recurrent spontaneous abortions have been attributed to deficient function of the corpus luteum,77,179,180,181 but the role of this mechanism remains conjectural because the diagnosis of luteal-phase defect is imprecise and controversial,65,182 and it is made in nonconceptive cycles.
Successful pregnancies in women with abetalipoproteinemia, in whom progesterone levels are low, would contradict the validity of defective luteal function as a cause of early pregnancy loss26; progesterone levels in subjects continuing pregnancy after luteectomy in the series of Csapo and coworkers149 and in women with normal pregnancies carefully studied at rest162 would suggest that levels of progesterone less than 10 ng/mL may not be abortifacient.179 Diagnosis and treatment of luteal defect as a cause of abortion should be made with caution pending verification of valid physiologic and diagnostic thresholds for progesterone levels in early pregnancy.181,183,184 Progesterone supplementation in the first trimester of pregnancy in women with a history of recurrent abortion or with threatened abortion may be reasonable, although not validated by study, when all three of the following are true: (1) normal ultrasonic landmarks for gestational age, (2) normal and normally rising levels of hCG, and (3) progesterone levels less than 10 ng/mL.
The cystic corpus luteum of pregnancy may reach a size warranting surveillance for neoplasm but generally regresses by the end of the first trimester. Pain and hemorrhage from corpora lutea of early pregnancy may mimic ectopic pregnancy and occasionally require surgical management. If luteectomy is necessary, allowance for a margin of safety for the luteoplacental shift at 7 weeks would dictate progesterone supplementation for pregnancies of 12 weeks or less.
CORPUS LUTEUM OF LATE PREGNANCY
Histology and Ultrastructure During Pregnancy
The size of the corpus luteum diminishes, owing principally to the loss of the central cavity. Luteinization of the theca layer declines, and granulosa cells are smaller. K-cells disappear, and vascularity decreases.185
Secretory Physiology During Pregnancy
After the increase seen over the first weeks of gestation, luteal secretion of progesterone144,146 and relaxin164 declines. Sampling of the ovarian vessels at cesarean section and the observation that progesterone levels rise in response to hCG administration postpartum confirm continued secretion of progesterone and relaxin by the corpus luteum at term and beyond.42,186,187,188,189 The contribution of the corpus luteum to overall progesterone secretion is small, relative to that of the placenta; this and the dispensability of the corpus luteum after the seventh week of gestation suggest that its continuance throughout pregnancy is a functional atavism. This is in contrast to the central role of the corpus luteum in the maintenance of pregnancy in species such as rodents, in which luteolysis itself is the initiator of parturition.
REFERENCES
Segaloff DL, Wang H, Richards JS: Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol 4:1856, 1990 |
|
Shi H, Segaloff DL: A role for increased lutropin/choriogonadotropin receptor receptor (LHR) gene transcription in the follitropin-stimulated induction of the LHR in granulosa cells. Mol Endocrinol 9:734, 1995 |
|
Ascoli M, Fanelli F, Segaloff DL: The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 23:141, 2002 |
|
World Health Organization Task Force on determination of the fertile period: Temporal relationships between ovulation and defined changes in the concentration of plasma estradiol-17β, luteinizing hormone, follicle-stimulating hormone, and progesterone. Am J Obstet Gynecol 139:886, 1981 |
|
Hoff JD, Quigley ME, Yen SSC: Hormonal dynamics at midcycle: A reevaluation. J Clin Endocrinol Metab 57:792, 1983 |
|
Johansson EDB, Wide L: Periovulatory levels of plasma progesterone and luteinizing hormone in women. Acta Endocrinol 62:82, 1969 |
|
Spirtos NJ, Foote C, Downing J, et al: Evaluation of the preovulatory rise of follicle stimulating hormone and progesterone in normally ovulating women of reproductive age. Int J Fertil 34:62, 1989 |
|
Aeado A-R, Landgren MB, Cekan Z, Diczfalusy E: Studies on the pattern of circulating steroids in the normal menstrual cycle. Acta Endocrinol 82:600, 1976 |
|
Ryan KJ, Petro Z: Steroid biosynthesis by human ovarian granulosa and theca cells. J Clin Endocrinol Metab 25:46, 1966 |
|
Eramaa M, Tuuri T, Hilden K, Ritvos O: Regulation of inhibin alpha and beta A-subunit messenger ribonucleic acid levels by chorionic gonadotropin and recombinant follicle-stimulating hormone in cultured human granulosa-luteal cells. J Clin Endocrinol Metab 79:1670, 1994 |
|
Fritz S, Kunz L, Dimitrijevic N, et al: Muscarinic receptors in human luteinized granulosa cells: Activation blocks gap junctions and induces the transcription factor early growth response factor-1. J Clin Endocrinol Metab 87:1362, 2002 |
|
Fritz S, Wessler I, Breitling R, et al: Expression of muscarinic receptor types in the primate ovary and evidence for non-neuronal acetylcholine synthesis. J Clin Endocrinol Metab 86:349, 2001 |
|
Adams EC, Hertig AT: Studies on the human corpus luteum II. J Cell Biol 41:716, 1969 |
|
Corner G: The histologic dating of the human corpus luteum of menstruation. Am J Anat 98:377, 1956 |
|
Crisp TM, Channing CP: Fine structural events correlated with progestin secretion during luteinization of rhesus monkey granulosa cells in culture. Biol Reprod 7:55, 1972 |
|
Gillira SW, Christensen AK, McLennan CE: Fine structure of the human menstrual corpus luteum at its stage of maximum secretory activity. Am J Anat 126:409, 1969 |
|
Crisp TM, Dessouky AD, Denys FR: The fine structure of the human corpus luteum of early pregnancy and during the progestational phase of the menstrual cycle. Am J Anat 127:37, 1970 |
|
Phillips HS, Hains J, Leung DN, Ferrara N: Vascular endothelial growth factor is expressed in rat corpus luteum. J Endocrinol 127:965, 1990 |
|
Gospodarowicz D, Cheng J, Lui G, et al: Corpus luteum angiogenic factor is related to fibroblast growth factor. Endocrinology 117:2383, 1985 |
|
Sugino N, Kashida S, Takiguchi S, et al: Expression of vascular endothelial growth factor and its receptors in the human corpus luteum during the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab 85:3919, 2000 |
|
Christensen LK, Stouffer RL: Isolation and culture of microvascular endothelial cells from the primate corpus luteum. Biol Reprod 55:1397, 1996 |
|
Wulff C, Dickson SE, Duncan WC, Fraser HM: Angiogenesis in the human corpus luteum: Simulated early pregnancy by HCG treatment is associated with both angiogenesis and vessel stabilization. Hum Reprod 16:2515, 2001 |
|
Christensen LK, Stouffer RL: Proliferation of microvascular endothelial cells in the primate corpus luteum during the menstrual cycle and simulated early pregnancy. Endocrinology 137:367, 1996 |
|
Hazzard TM, Christenson LK, Stouffer RL: Changes in expression of vascular endothelial growth factor and angiopoietin-1 and -2 in the macaque corpus luteum during the menstrual cycle. Mol Hum Reprod 6:993, 2000 |
|
Carr BR, Sadler RK, Rochelle DB, et al: Plasma lipoprotein regulation of progesterone biosynthesis by human corpus luteum tissue in organ culture. J Clin Endocrinol Metab 52:875, 1981 |
|
Parker CR, Illingworth DDR, Bissonnette J, Carr BR: Endocrine changes during pregnancy in a patient with homozygous familial hypobetalipoproteinemia. N Engl J Med 314:557, 1986 |
|
Illingworth DR, Corbin DK, Kemp ED, Keenan EJ: Hormone changes during the menstrual cycles in abetalipoproteinemia: Reduced luteal phase progesterone in a patient with homozygous hypobetalipoproteinemia. Proc Natl Acad Sci U S A 79:6685, 1982 |
|
Ashar SA, Menon KMJ: Receptor-mediated gonadotropin action in the ovary: Rat luteal cells preferentially utilize and are acutely dependent upon the plasma lipoprotein supplied sterols in gonadotropin stimulated steroid production. J Biol Chem 256:6548, 1981 |
|
Kallen CB, Billheimer JT, Summers SA: Steroidogenic acute regulatory protein (StAR) is a sterol transfer protein. J Biol Chem 273:26285, 1998 |
|
Pollack SF, Furth EE, Kallen CB, et al: Localization of the steroidogenic acute regulatory protein in human tissues. J Clin Endocrinol Metab 82:4243, 1997 |
|
Stocco DM: The role of the StAR protein in steroidogenesis: Challenges for the future. J Endocrinol 164:247, 2000 |
|
Sekar N, Garmey JC, Veldhuis JD: Mechanisms underlying the steroidogenic synergy of insulin and luteinizing hormone in porcine granulosa cells: Joint amplification of lipoprotein (LDL) receptor, steroidogenic acute regulatory (StAR) protein and cytochrome P450 side-chain cleavage (p450scc) enzyme. Mol Cell Endocrinol 159:25, 2000 |
|
Ghosh DK, Dunham WR, Sands RH, Menon KMJ: Regulation of cholesterol side-chain cleavage enzyme activity by gonadotropin in rat corpus luteum. Endocrinology 121:21, 1987 |
|
Devoto L, Kohen P, Gonzalez RR, et al: Expression of steroidogenic acute regulatory protein in the human corpus luteum throughout the luteal phase. J Clin Endocrinol Metab 86:5633, 2001 |
|
Enders AC: Cytology of the corpus luteum. Biol Reprod 8:158, 1973 |
|
Gemmell RT, Laychock SG, Rubin RP: Ultrastructural and biochemical evidence for a steroid-containing secretory organnelle in the perfused cat adrenal gland. J Cell Biol 72:209, 1977 |
|
Pearce RB, Crunshaw J, Holmes WN: The fine structure of the interrenal cells of the duck (Anas platyrhynchos) with evidence for the possible exocytotic release of steroids. Cell Tiss Res 183:203, 1977 |
|
Koninckx PR, Verhoeven G, Heyns W, et al: Biochemical characterization of peritoneal fluid in women during the menstrual cycle. J Clin Endocrinol Metab 51:1239, 1980 |
|
Gibson M, Samach A, Brumsted JR, Auletta FJ: Fate of peritoneal progesterone in the rabbit. Steroids 46:740, 1985 |
|
Auletta FJ, Kamps DL, Westney M, Gibson M: Luteolysis in the rhesus monkey: Ovarian venous estrogen, progesterone, and prostaglandin F2alpha metabolite. Prostaglandins 27:299, 1984 |
|
Filicori M, Butler J, CrowIcy WJR: Neuroendocrine regulation of the corpus luteum in the human. J Clin Invest 73:1638, 1984 |
|
Check J, Chase JH, Dietterich C: New approaches to the diagnosis and therapy of the luteinized unraptured follicle syndrome. Int J Fertil 30:29, 1986 |
|
Vega M, DeVoto L, Navarro V, et al: In vitro net progesterone production by human corpora lutea: Effects of human chorionic gonadotropin, dibutyryl adenosine 3′, 5′-monophosphate, cholers toxin, and forskolin. J Clin Endocrinol Metab 65:747, 1987 |
|
Fisch B, Margara RA, Winston RML, Hillier SG: Cellular basis of luteal steroidogenesis in the human ovary. J Endocrinol 122:303, 1989 |
|
O’Hara A, Mori T, Taii S, et al: Functional differentiation in steroidogenesis of two types of luteal cells isolated from mature human corpora lutea of menstrual cycle. J Clin Endocrinol Metab 65:1192, 1987 |
|
Niswender GD, Schwall RH, Fitz TA, et al: Regulation of luteal function in domestic ruminants: New concepts. Recent Prog Horm Res 41:101, 1985 |
|
Lei ZM, Chegini M, Ch V Rao: Quantitative cell composition of human and bovine corpora lutea on various reproductive states. Biol Reprod 44:148, 1991 |
|
Groff TR, Madhwa Raj HG, Talbert LM, Willis DL: Effects of neutralization of luteinizing hormone on corpus luteum function and cyclicity in macaca fascicularis. J Clin Endocrinol Metab 59:1054, 1984 |
|
McLachlan RI, Cohen NL, Vale WW, et al: The importance of luteinzing hormone in the control of inhibin and progesterone secretion by the human corpus luteum. J Clin Endocrinol Metab 68:1078, 1989 |
|
Casper RF, Yen SSC: Induction of luteolysis in the human with a long-acting analog of luteinizing hormone-releasing factor. Science 205:408, 1979 |
|
Casper RF, Sheehan KL, Yen SSC: Chorionic gonadotropin prevents lrf-agonist-induced luteolysis in the human. Contraception 21:471, 1980 |
|
Hutchison JS, Zeleznik AJ: The rhesus monkey corpus luteum is dependent on pituitary gonadotropin secretion throughout the luteal phase of the menstrual cycle. J Endocrinol 115:1780, 1984 |
|
Mais V, Kazer RR, Cetel NS, et al: The dependency of folliculogenesis and corpus luteum function on pulsatile gonadotropin secretion in cycling women using a gonadotropin-releasing hormone antagonist as a probe. J Clin Endocrinol Metab 62:1250, 1986 |
|
Messinis IE, Bergh T, Wide L: The importance of human chorionic gonadotropin support of the corpus luteum during human gonadotropin therapy in women with anovulatory infertility. Fertil Steril 50:31, 1988 |
|
Martin K, Santoro N, Hall J, et al: Management of ovulatory disorders with pulsatile gonadotropin-releasing hormone. J Clin Endocrinol Metab 71:1081A, 1990 |
|
Tsonis CG, Hillier SG, Baird DT: Production of inhibin bioactivity by human granulosa-lutein cells: Stimulation by LH and testosterone in vitro. J Endocrinol 112:R11, 1987 |
|
Davis SR, Krozowski Z, McLachlan RI, Burger HG: Inhibin gene expression in the human corpus luteum. J Endocrinol 115:R21, 1987 |
|
Basseti SG, Winters SJ, Keeping HS, Zeleznik AJ: Serum immunoreactive inhibin levels before and after luteectomy in the cynomolgus monkey (Macaca fascicularis). J Clin Endocrinol Metab 70:590, 1990 |
|
McClachlan RI, Robertson DM, Healy DL: Circulating immunoreactive inhibin levels during the normal human menstrual Cycle. J Clin Endocrinol Metab 65:954, 1987 |
|
Fraser HM, Lunn SF, Cowen GM, Saunders PT: Localization of inhibin/activin subunit mRNAs during the luteal phase in the primate ovary. J Mol Endocrinol 10:245, 1993 |
|
Eramaa M, Heikinheimo K, Tuuri T, et al: Inhibin/activin subunit mRNA expression in human granulosa-luteal cells. Mol Cell Endocrinol 92:R15, 1993 |
|
Stouffer RL, Dahl KD, Hess DL, et al: Systemic and intraluteal infusion of inhibin A or activin A in rhesus monkeys during the luteal phase of the menstrual cycle. Biol Reprod 50:888, 1994 |
|
McNatty KP, Hillier SG, Van den Boogard et al: Follicular development during the luteal phase of the human menstrual cycle. J Clin Endocrinol Metab 56:1022, 1983 |
|
Koninckx PR, Heyns WJ, Corvelyn PA: Delayed onset of luteinization as a cause of infertility. Fertil Steril 29:266, 1978 |
|
Gibson M: Clinical evaluation of luteal function. Semin Reprod Endocrinol 8:130, 1990 |
|
Kusuda M, Nakamura G, Matsukuma K, Kurano A: Corpus luteum insufficiency as a cause of nidatory failure. Acta Obstet Gynecol Scand 62:199, 1983 |
|
Tresch DD, Halverson G, Blick M, Keelan MH: Ovarian (corpus luteum) hemorrhage during anticoagulation therapy. Ann Intern Med 88:642, 1978 |
|
Brewer JI: Studies of the human corpus luteum. Am J Obstet Gynecol 44:1048, 1942 |
|
Guraya SS: Morphology, histochemistry, and biochemistry of human ovarian compartments and steroid hormone synthesis. Physiol Rev 51:785, 1971 |
|
Richardson MC: Hormonal control of ovarian luteal cells. Oxford Rev Reprod Biol 8:321, 1986 |
|
Soules MR, Steiner RA, Clifton DK, et al: Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab 58:378, 1984 |
|
Fraser HM, Dickson SE, Lunn SF, et al: Suppression of luteal angiogenesis in the primate after neutralization of vascular endothelial growth factor. Endocrinology 141:995, 2000 |
|
Wang HZ, Lu SH, Han XJ, et al: Control of inhibin production by dispersed human luteal cells in vitro. Reprod Fertil Dev 4:67, 1992 |
|
Ropert JF, Quigley ME, Yen SSC: Endogenous opiates modulate pulsatile luteinizing hormone release in humans. J Clin Endocrinol Metab 52:583, 1981 |
|
Gibson M, Nakajima ST, McAuliffe TL: Short-term modulation of gonadotropin secretion by progesterone during the luteal phase. Fertil Steril 55:522, 1991 |
|
Soules MR, Clifton DK, Steiner RA: The corpus luteum: Determinants of progesterone secretion in the normal menstrual cycle. Obstet Gynecol 71:659, 1988 |
|
Soules MR, Clifton DK, Cohen NL, et al: Luteal phase abnormal gonadotropin and secretion patterns. J Clin Endocrinol Metab 69:813, 1989 |
|
Baird DT, Backstrom T, McNeilly AS: Effect of enucleation of the corpus luteum at different stages of the luteal phase of the human menstrual cycle on subsequent follicular development. J Reprod Fertil 70:615, 1984 |
|
Yamamoto M, Imai M, Otani H, Nakano R: Serum levels of inhibin A and inhibin B in women with normal and abnormal luteal function. Obstet Gynecol 89:773, 1997 |
|
Balasch J, Creus M, Fabregues F, et al: Midluteal immunoreactive alpha inhibin serum concentrations as markers of luteal phase deficiency. Hum Reprod 11:2591, 1996 |
|
Andoh K, Mizunuma H, Hasegawa Y, et al: Association of immunoreactive-inhibin levels with luteal function. Horm Res 37(suppl 1):37, 1992 |
|
Wiley CA, Esterly JR: Observations on the human corpus luteum: Histochemical changes during development and involution. Am J Obstet Gynecol 125:514, 1976 |
|
De-Kretser DM, Robertson DM: The isolation and physiology of inhibin and related proteins. Biol Reprod 40:33, 1989 |
|
Nakajima ST, McLachlan RI, Cohen NL, et al: The immunoreactive inhibin secretion pattern in midluteal phase: Relationships with leutinizing hormone and progesterone. Clin Endocrinol 33:709, 1990 |
|
Lahlou N, Chabbert-Buffet N, Christin-Maitre S, et al: Main inhibitor of follicle stimulating hormone in the luteal-follicular transition: inhibin A, estradiol, or inhibin B? Hum Reprod 14:1190, 1999 |
|
Welt CK, Martin KA, Taylor AE, et al: Frequency modulation of follicle-stimulating hormone (FSH) during the luteal-follicular transition: Evidence for FSH control of inhibin B in normal women. J Clin Endocrinol Metab 82:2645, 1997 |
|
Maruncle M, Casper RF: The effect of luteal phase estrogen antagonism on luteinizing hormone pulsatility and luteal function in women. J Clin Endocrinol Metab 64:148, 1987 |
|
Hutchison JS, Nelson PD, Zeleznik AJ: Effects of different gonadotropin pulse frequencies on corpus luteum function during the menstrual cycle of rhesus monkeys. Endocrinology 119:1964, 1986 |
|
Zeleznik AJ, Little-Ihrig LL: Effect of reduced luteinizing hormone concentrations on corpus luteum function during the menstrual cycle of rhesus monkeys. Endocrinology 125:2237, 1990 |
|
Friedrich F, Kemeter P, Salzer H, Breitenecker G: Ovulation inhibition with human chorionic gonadotrophin. Acta Endocrinol 78:332, 1975 |
|
Halme J, Ikonen M, Rutanen EM, Seppala M: Gonadotropin receptors of human corpus luteum during menstrual cycle and pregnancy. Am J Obstet Gynecol 13:728, 1978 |
|
Bramley TA, Stirling D, Swanston IA, et al: Specific binding sites for gonadotrophin-releasing hormone, LH/chorionic gonadotrophin, low-density lipoprotein, prolactin and FSH in homogenates of human corpus luteum: II. Concentrations throughout the luteal phase of the menstrual cycle and early pregnancy J Endocrinol 113:317, 1987 |
|
Cameron JL, Stouffer RL: Gonadotropin receptors of the primate corpus luteurn: II. Changes in available luteinizing hormone and chorionic gonadotropin-binding sites in macaque luteal membranes during the nonfertile menstrual cycle Endocrinology 110:2068, 1982 |
|
Auletta FJ, Schofield M, Abae M: The mechanisms controlling luteolysis in non-human primates and women. Semin Reprod Endocrinol 8:122, 1990 |
|
Chung PH, Sandhoff TW, McLean MP: Hormone and prostaglandin F2alpha regulation of messenger ribonucleic acid encoding steroidogenic acute regulatory protein in human corpora lutea. Endocrine 8:153, 1998 |
|
Auletta FJ, Flint APF: Mechanisms controlling corpus luteum function in sheep, cows, nonhuman primates, and women especially in relation to the time of luteolysis. Endocr Rev 9:1, 1988 |
|
Goding JR: The demonstration that PGF2α is the uterine luteolysin in the ewe. J Reprod Fertil 38:261, 1974 |
|
McCracken J: Prostaglandin F2α and corpus luteum regression. Ann N Y Acad Sci 180:456, 1971 |
|
Challis JRG, Calder AA, Dilley S, et al: Production of prostaglandins E and F2α by corpora lutea, corpora albicantes and stroma from the human ovary. J Endocrinol 68:401, 1976 |
|
Lahav M, Freud A, Lindner HR: Abrogation by prostaglandin F2α of LH-stimulated cyclic AMP accumulation in isolated rat corpora lutea of pregnancy. Biochem Biophysical Res Comm 68:1294, 1976 |
|
Grinwich DL, Ham EA, Hichens M, Behrman HR: Binding of human chorionic gonadotropin and response of cyclic nucleotides to luteinizing hormone in luteal tissue from rats treated with prostaglandin F2α. Endocrinology 98:146, 1975 |
|
Hichens M, Grinwich DL, Behrman HR: PGF2α-induced loss of corpus luteum gonadotroph in receptors. Prostaglandins 7:449, 1974 |
|
Sotrel G, Helvacioglu A, Dowers S, et al: Mechanism of luteolysis: Effect of estradiol and prostaglandin F2 on corpus luteum luteinizing hormone human chorionic gonadotropin receptors and cyclic nucleotides in the rhesus monkey. Am J Obstet Gynecol 139:134, 1981 |
|
Williams MT, Roth MS, Marsh MJ, Lemaire WJ: Inhibition of human chorionic gonadotropin-induced progesterone synthesis by estradiol in isolated human luteal cells. J Clin Endocrinol Metab 48:437, 1979 |
|
Sandhoff TW, McLean MP: Repression of the rat steroidogenic acute regulatory protein gene by PGF2alpha is modulated by the negative transcription factor DAX-1. Endocrine 10:83, 1999 |
|
Bennegard B, Dennefors B, Hamberger L: Interaction between catecholamines and prostaglandin F2α in human luteolysis. Acta Endocrinol 106:532, 1984 |
|
Andreoli C: Corpus luteum activity after hysterectomy in women. Acta Endocrinol 50:65, 1965 |
|
Anderson LL: Effects of hysterectomy and other factors on luteal function. In Greep RO (ed): Handbook of Physiology, Section 7: Endocrinology. Baltimore, Williams & Wilkins, 1973 |
|
Beling CG, Marcus SL, Markham MS: Functional activity of the corpus luteum following hysterectomy. J Clin Endocrinol 30:30, 1970 |
|
Vijayakumar R, Waiters WAW: Human luteal tissue prostaglandins, 17Β-estradiol, and progesterone inrelation to the growth and senescence of the corpus luteum. Fertil Steril 39:298, 1983 |
|
Pharriss BB, Tillson SA, Erickson RR: Prostaglandins in luteal function. Recent Prog Horm Res 28:51, 1972 |
|
Auletta FJ, Speroff L, Caldwell BV: Prostaglandin F2α induced steroidogenesis and luteolysis in the primate corpus luteum. J Clin Endocrinol Metab 36:405, 1973 |
|
Johnson MS, Ottobre AC, Ottobre JS: Prostaglandin production by corpora luteal of rhesus monkeys: Characterization of incubation conditions and examination of putative regulators. Biol Reprod 39:839, 1988 |
|
Shutt DA, Clarke AH, Fraser IS, et al: Changes in concentration of prostaglandin F and steroids in human corpora lutea in relation to growth of the corpus luteum and luteolysis. J Endocrinol 71:453, 1976 |
|
Auletta FJ, Cadwell BB, Speroff L: Estrogen-induced luteolysis in the rhesus monkey: Reversal with indomethacin. Prostaglandins 11:745, 1976 |
|
Wentz AC, Jones GS: Transient luteolytic effect of prostaglandin F2α in the human. Obstet Gynecol 42:172, 1973 |
|
Leader A, Bygdeman M, Eneroth P, et al: The effect of infusions with two analogues of prostaglandin F2α on corpus luteum function. Adv Prostaglandin 2:679, 1976 |
|
Gibson M, Auletta FJ: Effect of prostaglandin synthesis inhibition on human corpus luteum function. Prostaglandins 31:1023, 1986 |
|
Sargent EL, Baughman WL, Novy MJ, Stouffer RL: Intraluteal infusion of a prostaglandin synthesis inhibitor, sodium meclofenamate, causes premature luteolysis in rhesus monkeys. Endocrinology 123:2261, 1988 |
|
Zelinski-Wooten MB, Stouffer RL;: Intraluteal infusions of prostaglandins of the E, D, I, and A series prevent PGF2α-induced, but not spontaneous, luteal regression in rhesus monkeys. Biol Reprod 43:507, 1990 |
|
Bennegard B, Hahlin M, Hamberger L: Luteotrophic effects of prostaglandins I2 and D2 on isolated human corpus luteum. Fertil Steril 54:459, 1990 |
|
Johansson EDB: Inhibition of the corpus luteum function in women taking large doses of diethylstilbestrol. Contraception 8:27, 1973 |
|
Karsch FJ, Sutton GP: An intra-ovarian site for the luteolytic action of estrogen in the rhesus monkey. Endocrinology 98:553, 1976 |
|
Gore BZ, Caldwell BB, Speroff L: Estrogen-induced human luteolysis. J Clin Endocrinol Metab 36:615, 1973 |
|
Stouffer RL, Nixon WE, Hodgen GD: Estrogen inhibition of basal and gonadotropin-stimulated progesterone production by rhesus monkey luteal cells in vitro. Endocrinology 101:1157, 1977 |
|
Butler WR, Hotchkiss J, Knobil E: Functional luteolysis in the rhesus monkey: Ovarian estrogen and progesterone during the luteal phase of the menstrual cycle. Endocrinology 96:1509, 1975 |
|
Auletta FJ, Agins H, Scommegna A: Prostaglandin F mediation of the inhibitory effect of estrogen on the corpus luteum of the rhesus monkey. Endocrinology 103:1183, 1978 |
|
Blumenfeld Z, Nahhas F: Luteal dysfunction in ovulation induction: The role of repetitive human chorionic gonadotropin supplementation during the luteal phase. Fertil Steril 50:403, 1988 |
|
Messinis IE, Templeton A, Baird DT: Luteal phase after ovarian hyperstimulation. Br J Obstet Gynaecol 94:345, 1987 |
|
Yuan W, Giudice LC: Programmed cell death in human ovary is a function of follicle and corpus luteum status. J Clin Endocrinol Metab 82:3148, 1997 |
|
Hunot S, Flavell RA: Death of a monopoly? Science 292:865, 2001 |
|
Sugino N, Suzuki T, Kashida S, et al: Expression of Bcl-2 and Bax in the human corpus luteum during the menstrual cycle and in early pregnancy: Regulation by human chorionic gonadotropin. J Clin Endocrinol Metab 85:4379, 2000 |
|
Lenton EA, Landgren B-M, Sexton L: Normal variation in the length of the luteal phase of the menstrual cycle: Identification of the short luteal phase. Br J Obstet Gynaecol 91:685, 1984 |
|
Sherman BM, Korenman SG: Measurement of plasma LH, FSH, estradiol and progesterone in disorders of the human menstrual cycle: The short luteal phase. J Clin Endocrinol Metab 38:89, 1974 |
|
Strott CA, Cargille CM, Ross GT, Lipsett MB: The short luteal phase. J Clin Endocrinol Metab 30:246, 1970 |
|
Soules MR, Steiner RA, Clifton DK, Bremner WJ: Abnormal patterns of pulsatile luteinizing hormone in women with luteal phase deficiency. Obstet Gynecol 63:626, 1984 |
|
Soules MR, Clifton DK, Bremner WJ, Steiner RA: Corpus luteum insufficiency induced by a rapid gonadotropin-releasing hormone-induced gonadotropin secretion pattern in the follicular phase. J Clin Endocrinol Metab 65:457, 1987 |
|
Stouffer RL, Hodgen GD: Induction of luteal phase defects in rhesus monkeys by follicular fluid administration at the onset of the menstrual cycle. J Clin Endocrinol Metab 51:669, 1980 |
|
Dizerega GS, Hodgen GD: Luteal phase dysfunction in infertility: A sequel to aberrant folliculogenesis. Fertil Steril 35:489, 1981 |
|
Aksel S, Schomberg DW, Hammond CB: Prostaglandin F2α levels in human ovarian plasma in pregnancy and in a case of Halban’s disease. Obstet Gynecol 52:421, 1978 |
|
Nelson WW, Greene RR: Some observations on the histology of the human ovary during pregnancy. Am J Obstet Gynecol 76:66, 1958 |
|
Gillman J, Stein HB: The human corpus luteum of pregnancy. Surg Gynecol Obstet 72:129, 1941 |
|
Branch CM, Collins PO, Kilpatrick MJ, Collins WP: The effect of conception on the concentration of urinary oestrone-3-glucuronide, LH/hCG and pregnanediol-3 alphaglucuronide. Acta Endocrinol 93:228, 1980 |
|
Yoshimi T, Strott CA, Marshall JR, Lipsett MB: Corpus luteum function in early pregnancy. J Clin Endocrinol Metab 29:225, 1969 |
|
Lenton EA, Woodward AJ: The endocrinology of conception cycles and implantation in women. J Reprod Fertil 36(suppl):1, 1988 |
|
Soules MR, Hughes CL, Aksel S, et al: The function of the corpus luteum of pregnancy in ovulatory dysfunction and luteal phase deficiency. Fertil Steril 36:31, 1981 |
|
Balmaceda JP, Valenzuela G, Eddy CA, Asch RH: Effects of hCG on prostaglandin synthesis and function of corpus luteum. Obstet Gynecol 57:505, 1981 |
|
Ottobre JS, Houmard BS, Ottobre AC: Luteal production of steroids and prostaglandins during simulated early pregnancy in the primate: Differential regulation of steroid production by chorionic gonadotropin. Biol Reprod 41:393, 1989 |
|
Csapo AI, Pulkkinen M: Indispensability of the human corpus luteum in the maintenance of early pregnancy luteectomy evidence. Obstet Gynecol Surv 33:69, 1978 |
|
Nakajima ST, Nason FG, Badger GJ, Gibson M: Luteal progesterone production in early pregnancy. Fertil Steril 55:516, 1991 |
|
Mishell DR, Thorneycroft IH, Nagata Y, et al: Serum gonadotropin and steroid patterns in early human gestation. Am J Obstet Gynecol 117:631, 1973 |
|
Csapo AI, Pulkkinen MO, Kaihola HL: The relationship between the timing of luteectomy and the incidence of complete abortions. Am J Obstet Gynecol 118:985, 1974 |
|
Csapo AI, Pulkkinen MO, Kaihola HL: The effect of estradiol replacement therapy on early pregnant luteectomized patients. Am J Obstet Gynecol 117:987, 1973 |
|
Csapo AI, Pulkkinen MO, Wiest WG: Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am J Obstet Gynecol 115:759, 1973 |
|
Croxatto HB, Salvatierra AM, Croxatto HD, Spitz IM: Variable effects of RU 486 on endometrial maintenance in the luteal phase extended by exogenous hCG. Clin Endocrinol 31:15, 1989 |
|
Wehrenberg WB, Dierschke DJ, Wolf RC, Vernon MW: Effect of intrauterine and intramuscular administration of human chorionic gonadotropin on corpus luteum function in cyclic rhesus monkeys. Biol Reprod 23:10, 1980 |
|
Neill JD, Knobil E: On the nature of the initial luteotropic stimulus of pregnancy in the rhesus monkey. Endocrinology 90:34, 1972 |
|
Ottobre JS, Stouffer RL: Persistent versus transient stimulation of the macaque corpus luteum during prolonged exposure to human chorionic gonadotropin: A function of age of the corpus luteurn. Endocrinology 114:1275, 1984 |
|
Min L, Ascoli M: Effect of activating and inactivating mutations on the phsophorylation and trafficking of the human lutropin/choriogonadotropin receptor. Mol Endocrinol 14:1797, 2000 |
|
Stovall TG, Ling FW, Carson SA, Buster JE: Serum progesterone and uterine curettage in differential diagnosis of ectopic pregnancy. Fertil Steril 57:456, 1992 |
|
Ottobre JS, Ottobre AC, Stouffer RL: Changes in available gonadotropin receptors in the corpus luteum of the rhesus monkey during simulation early pregnancy. Endocrinology 115:198, 1984 |
|
Nakajima ST, McAuliffe T, Gibson M: The 24-hour pattern of the levels of serum progesterone and immunoreactive human chorionic gonadotropin in normal early pregnancy. J Clin Endocrinol Metab 71:345, 1990 |
|
Stewart DS, Celniker AC, Taylor CA, et al: Relaxin in the peri-implantation period. J Clin Endocrinol Metab 70:1771, 1990 |
|
Quagliarello J, Szlachter N, Steinetz BG, et al: Serial relaxin concentrations in human pregnancy. Am J Obstet Gynecol 135:43, 1979 |
|
Duffy DM, Hutchison JS, Stewart DR, Stouffer RL: Stimulation of primate luteal function by recombinant human chorionic gonadotropin and modulation of steroid, but not relaxin production by an inhibitor of 3 beta-hydroxysteroid dehydrogenase during simulated early pregnancy. J Clin Endocrinol Metab 81:2307, 1996 |
|
Duffy DM, Stouffer RL, Stewart DR: Dissociation of relaxin and progesterone secretion from the primate corpus luteum by acute administration of a 3 beta-hydroxysteroid dehydrogenase inhibitor during the menstrual cycle. Biol Reprod 53:447, 1995 |
|
Quagliarello J, Goldsmith LT, Steinetz BG, Lustig DS: Induction of relaxin secretion in nonpregnant women by human chorionic gonadotropin. J Clin Endocrinol Metab 51:74, 1980 |
|
Johnson MR, Abbas AA, Allman AC, et al: The regulation of plasma relaxin levels during human pregnancy. J Endocrinol 142:261, 1994 |
|
Glock JL, Nakajima ST, Stewart DR, et al: The relationship of corpus luteum volume to relaxin, estradiol, progesterone, 17-hydroxyprogesterone and human chorionic gonadotropin levels in early normal pregnancy. Early Pregnancy 1:206, 1995 |
|
Unemori EN, Erikson ME, Rocco SE, et al: Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod 14:800, 1999 |
|
Maclennan AH: The role of relaxin in human reproduction. Clin Reprod Fertil 2:77, 1983 |
|
Bani D: Relaxin: A pleiotropic hormone. Gen Pharmacol 28:13, 1997 |
|
Bogic LV, Mandel M, Bryant-Greenwood GD: Relaxin gene expression in human reproductive tissues by in situ hybridization. J Clin Endocrinol Metab 80:130, 1995 |
|
Steinetz BG, Whitaker PG, Edwards JR: Maternal relaxin concentrations in diabetic pregnancy. Lancet 340:752, 1992 |
|
Arthur ID, Anthony FW, Adams S, Thomas EJ: Serum relaxin and the major endometrial secretory proteins in in-vitro fertilization and down-regulated hormone-supported and natural cycle frozen embryo transfer. Hum Reprod 11:88, 1996 |
|
Goldsmith LT, Weiss G, Steinetz BG: Relaxin and its role in pregnancy. Endocrinol Metab Clin N Am 24:171, 1995 |
|
Johnson MR, Abdalla H, Allman AC, et al: Relaxin levels in ovum donation pregnancies. Fertil Steril 56:59, 1991 |
|
Hubinont CJ, Thomas C, Schwers JF: Luteal function in ectopic pregnancy. Am J Obstet Gynecol 156:669, 1987 |
|
Daya S, Ward S, Burrows E: Progesterone profiles in luteal phase defect cycles and outcome of progesterone treatment in patients with recurrent spontaneous abortion. Am J Obstet Gynecol 158:225, 1988 |
|
Balasch J: The significance of luteal phase defect. Hum Reprod 1:145, 1986 |
|
Daya S: Efficacy of progesterone support for pregnancy in women with recurrent miscarriage: A meta-analysis of controlled trials. Br J Obstet Gynaecol 96:275, 1989 |
|
McNeely MJ, Soules MR: The diagnosis of luteal phase deficiency: A critical review. Fertil Steril 50:1, 1988 |
|
Goldstein P, Berrier J, Rosen S, et al: A meta-analysis of randomized control trials of progestational agents in pregnancy. Br J Obstet Gynaecol 96:265, 1989 |
|
Macdonald RR: Does treatment with progesterone prevent miscarriage? Br J Obstet Gynaecol 96:257, 1989 |
|
Visfeldt J, Starup J: Histology of the human corpus luteum of early and late pregnancy. Acta Pathol Microbiol Scand 83:669, 1975 |
|
Lemaire WJ, Conly PW, Moffett A, Cleveland WW: Plasma progesterone secretion by the corpus luteum of term pregnancy. Am J Obstet Gynecol 108:132, 1970 |
|
Weis G, O’Byrne EM, Hochman JA, et al: Secretion of progesterone and relaxin by the human corpus luteum at midpregnancy and at term. Obstet Gynecol 50:679, 1977 |
|
Mikhail G, Allen WM: Ovarian function in human pregnancy. Am J Obstet Gynecol 99:308, 1967 |
|
Lemaire WJ, Conly PW, Moffett A, et al: Function of the human corpus luteum during the puerperium: Its maintenance by exogenous human chorionic gonadotropin. Am J Obstet Gynecol 110:612, 1971 |